Why Pharma Can’t Afford ‘Good Enough’ AI
Generic AI tools like ChatGPT and Microsoft Copilot are rapidly being adopted across pharmaceutical organizations, with teams expecting them to handle complex use cases involving internal medical data. Yet despite their promise, these tools frequently fall short in pharmaceutical, biotech, and medtech environments. While they excel at general business tasks, they lack the precision and specialized capabilities that life sciences organizations require for their critical knowledge work.
The High Stakes of Pharmaceutical Knowledge Work
When developing regulatory submissions, analyzing clinical trial data, supporting market access strategies, or ensuring safety compliance, precision in knowledge work is critical and often seen as the key problem with AI as the results look great but in face are not reflecting evidences and real date in a precise and accurate way.
Why Generic AI Falls Short in Life Sciences
The Data Source Transparency Problem
ChatGPT and Copilot operate as “black boxes” – you get responses, but you can’t verify where the information came from or whether it’s based on current, approved sources. In pharmaceutical knowledge work, this opacity creates immediate trust barriers. Regulatory teams need clear audit trails, medical affairs requires validated references, and safety organizations must trace every conclusion back to its source.
The Consistency Challenge
Generic AI tools require extensive prompt engineering to achieve even moderate consistency in pharmaceutical contexts. Different team members asking similar questions get different results, making it impossible to build reliable workflows around these tools. This inconsistency becomes problematic when teams need systematic, repeatable processes for regulatory submissions, safety reviews, or market access strategies.
Missing Pharmaceutical Context
While you can provide context through prompts, generic AI lacks the deep pharmaceutical intelligence needed for specialized use cases. It doesn’t understand that certain adverse events require immediate flagging, that bioavailability differences have regulatory implications, or that protocol deviations need specific handling. This context gap means teams spend more time correcting and validating AI outputs than they save by using them.
The “Current Information” Gap
Pharmaceutical work requires the most up-to-date regulatory guidance, safety data, and clinical findings. Generic AI models are trained on static datasets and can’t integrate real-time pharmaceutical data sources, creating a fundamental limitation for organizations that need current, approved materials in their analyses.
The OneRay.ai Difference: Precision AI for Life Sciences
OneRay.ai was designed specifically to deliver the precision that pharmaceutical organizations require. Rather than trying to force general-purpose tools into specialized workflows, we built AI that understands pharmaceutical complexity and delivers the accuracy and reliability that life sciences teams demand.
Transparent Referencing for Complete Precision
Every OneRay.ai response includes complete source transparency with validated references. Our automated referencing engine provides credibility scoring and source validation, ensuring that medical affairs teams, regulatory professionals, and safety organizations can trace every piece of information back to its original, approved source.
Image illustrating the precision referencing of multiple reference points within a document such as a publication.
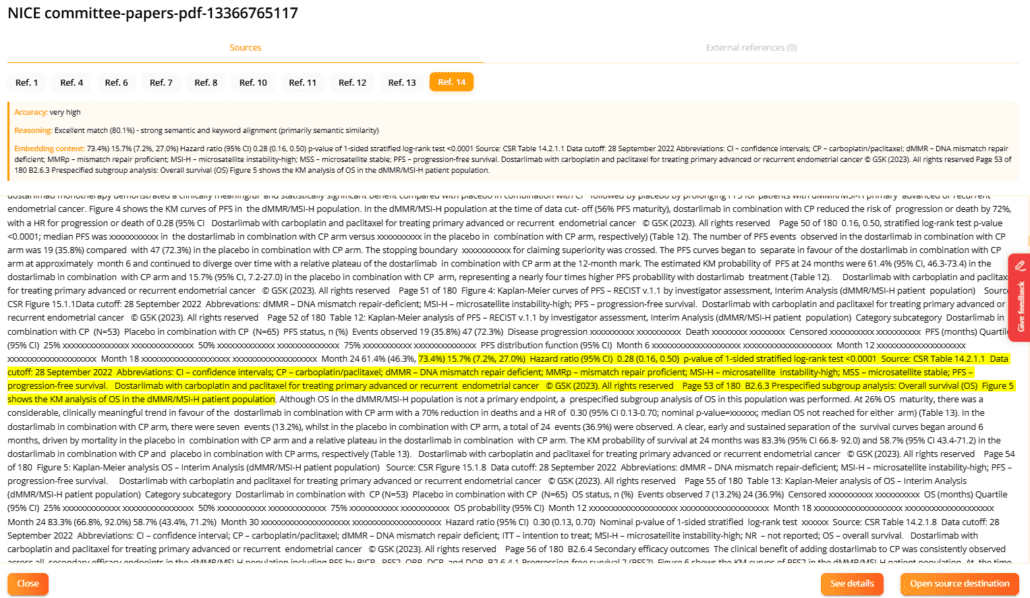
Image illustrating the highlighting of a specific text passage in yellow that has been used as a reference for the content generated.
-
Pre-trained Pharmaceutical Intelligence
Instead of requiring complex prompt engineering, OneRay.ai comes with configurable agent stacks designed specifically for pharmaceutical workflows. From healthcare professional communications to systematic literature reviews, each agent understands the nuances of its domain and delivers consistent, precise results.
-
Scientific Precision with Pharmaceutical Context
Our tailored data models understand the hierarchical importance of pharmaceutical information. The system automatically prioritizes safety signals, recognizes regulatory implications, and applies pharmaceutical-specific logic that generic AI simply cannot replicate through prompting alone.
-
Real-time Integration with Approved Sources
OneRay.ai integrates with real-time pharmaceutical data sources and maintains access to current regulatory guidance, ensuring that all analyses are based on the most recent approved materials. This real-time integration eliminates the “current information” gap that limits generic AI implementations.
-
Compliant Data Access from Day One
Unlike generic solutions where compliance is an afterthought, OneRay.ai was built with pharmaceutical-grade data security and compliance requirements. The system includes export functionalities with multiple format options and confidentiality clauses, ensuring that all work meets the stringent requirements pharmaceutical organizations demand.
-
Advanced Document Processing
OneRay.ai handles multiple files simultaneously (up to 5 files) with persistent storage and maintains searchable conversation history—capabilities that are essential for complex pharmaceutical analyses but challenging to achieve consistently with generic AI tools.
Precision That Transforms Pharmaceutical Knowledge Work
The difference between generic AI and precision AI lies in understanding that pharmaceutical organizations can’t afford approximations. When regulatory teams analyze submission requirements, when medical affairs teams review clinical data, when safety teams monitor adverse events—the work demands precision, not “good enough” responses.
The Strategic Choice: Generic vs. Precision
Generic AI tools like ChatGPT and Copilot will continue to evolve and improve, but they will continue to be designed for broad, general-purpose applications. The fundamental architecture decisions that make them excel at general business tasks also create the limitations that prevent effective use in specialized pharmaceutical environments.
The question for pharmaceutical organizations isn’t whether AI will transform their knowledge work—it’s whether they’ll achieve that transformation through generic tools that require constant workarounds or precision AI designed specifically for their industry’s exacting standards.
Purpose-Built for Pharmaceutical Excellence
Every day spent trying to make generic AI work in pharmaceutical contexts is a day not spent on the strategic initiatives that could accelerate treatment development and improve patient outcomes. The precision requirements aren’t just technical preferences—they’re fundamental requirements for the knowledge work that ultimately determines how quickly and safely new treatments reach patients.
Purpose-built precision AI doesn’t just add artificial intelligence to pharmaceutical processes—it transforms them with the accuracy, transparency, and reliability that pharmaceutical teams need to excel in their critical work.
Ready to experience precision AI designed for life sciences?
OneRay.ai delivers the specialized pharmaceutical intelligence that transforms knowledge work across medical affairs, regulatory, safety, and market access teams.
